Patient and Public Involvement in Research
On this web page, we use the term Patient and Public Involvement (PPI) to describe the involvement of patients and members of the public in the research process. This terminology is widely used in the UK and parts of Europe. However, we acknowledge that in other countries, different terms may be preferred. For example, “patient engagement” is commonly used in the United States and Canada, while “consumer and community involvement” is often used in Australia. You may also encounter related terms such as community engagement, stakeholder involvement, or co-production, all of which reflect a shared commitment to involving people with lived experience in research.
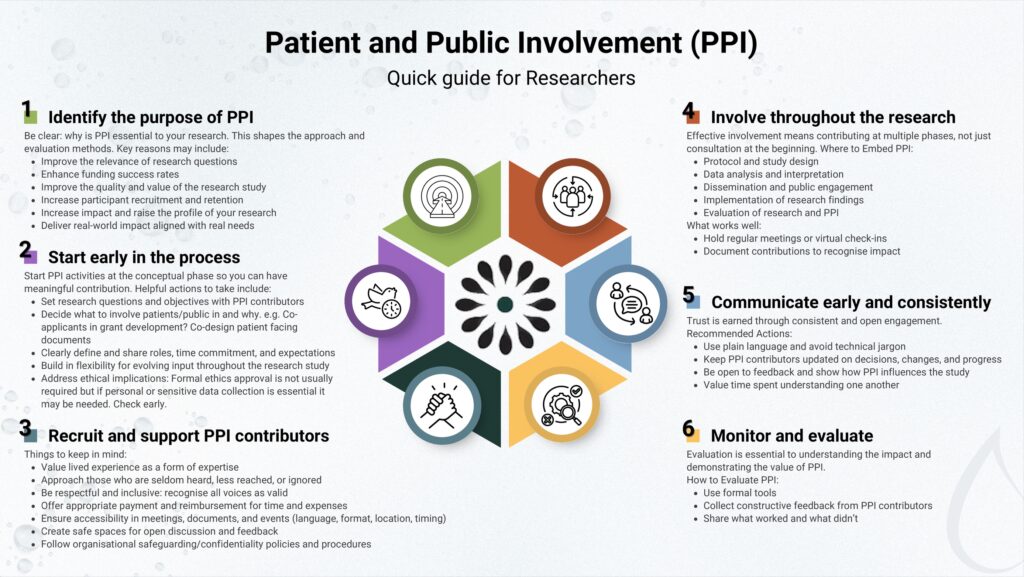
Quick Guide
What Is PPI?
PPI means research is done with you, not about you. Instead of researchers making all the decisions on their own, they work alongside people like you to make sure the research truly reflects what matters in real life.
You do not need a background in science or research to participate as you bring valuable insight simply by sharing your experience. People of all ages and backgrounds are welcome, especially those with lived experience of a health condition or those from communities that are often underrepresented in research.
Why should you get involved?
- Your lived experience brings real life insight to research
- You can help improve healthcare and ensure studies reflect what matters most to patients.
- Be part of change and influence how research is conducted
- Gain new experiences and knowledge
- Getting involved in research can be rewarding and impactful
What can you do as a PPI contributor?
- Help identify research questions
- Review study materials and patient-facing documents
- Advise on how to recruit participants
- Join focus groups
- Help interpret and share research findings
And much more! Your research team will walk you through all the details of your role, so you’ll know exactly what to expect and how you’ll be contributing.
How will you be supported?
- You’ll be part of a team that values your input
- You’ll be offered support or training
- You may be offered payment for your time
- Travel and other expenses are usually covered so you’re not out of pocket
- Your involvement can fit around your life and health
- Whether it’s online, in person, or by phone, you can take part in the way that feels right for you
Where to look for opportunities?
- Research hospitals and universities often conduct studies involving PPI
- Social media and online forums may advertise PPI opportunities
- Your healthcare team may know of ways for you to get involved in research
- Check out the Resources section below for useful links to PPI opportunities around the world
Case Studies
Background:
Multiple Myeloma (MM) is a blood cancer characterised by the overproduction of monoclonal paraprotein (M-protein), which is central to both diagnosis and ongoing disease monitoring. Measuring M-protein typically requires laboratory testing carried out at a central laboratory, meaning patients often need to travel to attend frequent phlebotomy appointments. While these may sometimes coincide with other clinic visits, patients are frequently required to make additional trips, which can be burdensome. The overarching aim of this study is to explore whether alternative microsampling methods could be used in place of standard phlebotomy.
Aims:
We aim to collaborate with people with lived experience of MM to help shape a microsampling pathway that truly fits their needs and circumstances. Through meaningful collaboration with these individuals, we hope to gain valuable insights into their perspectives. This understanding is key to shaping a microsampling service that is not only scientifically sound but also practical and supportive of MM patients.
Methods:
We have formed a national PPI panel of individuals with lived experience to gather a broad range of perspectives on the implementation of a remote monitoring scheme and to support the trial throughout its various stages. To make certain representatives with different socio-economic and demographic ethnic profiles were included, we contacted Myeloma UK support group leaders and snowball sampling tactics were used to identify a wide range of patient and public contributors.
So far, two online workshops have taken place. A third, in-person workshop is planned to include those who may be digitally excluded. The first session introduced the project and outlined its aims. Ahead of the meeting, mock sampling kits were sent to participants, containing five different potential microsampling devices and food colouring to mimic blood. During the session, we led an interactive demonstration of the sample collection process. Participants provided valuable feedback and shared their preferences, which helped influence the choice of the microsampling device for the trial.
In the second workshop, the panel contributed to developing the study protocol and reviewed patient-facing materials such as consent forms and questionnaires. Their feedback helped ensure that these documents were clear and user friendly.
Impact in research:
PPI input proved invaluable in refining the study approach. As the trial progresses into its second phase, we are committed to expanding PPI involvement so that patients continue to play a central role in shaping this research. Our next focus will be to reach out to community leaders and build trust within various communities to help ensure a wide range of voices are heard.
PCSIG Webinar

The IN-HOME study assesses the feasibility and acceptability of using a whole-blood creatinine point-of-care testing device (NOVA Biomedical StatSensor® Xpress) in the home, to monitor patient kidney function whilst receiving anticancer treatments that are potentially toxic to the kidneys.
Improved monitoring of kidney function could potentially lead to earlier detection of adverse renal events and improve patient outcomes. This could also alleviate the risk of patients with reduced kidney function going on to clinical trials.
This webinar covers the end-to-end process that the team has gone through from initial idea, device selection and working with patients and healthcare professional to design an appropriate study to evaluate the feasibility of using the technology in the patient’s home. Key learnings to date from the practical delivery of a research study into the UK NHS are also shared.
Attending a phlebotomy appointment can cause high levels of anxiety, especially in patients with learning disabilities. Alternative blood collection methods are now available and offer reasonable adjustments to phlebotomy for many patient groups.
Analytical verification of blood tests from capillary samples vs standard venepuncture samples was done using our Beckman Coulter AU580 and DXI800 automated analyzers. A standard operating procedure has now been established as part of an end-to-end service, including electronic requesting, patient phlebotomy within a primary care setting according to clinical requirements, to provide reasonable adjustments for our local patients with learning disabilities. This service improvement allows patients to provide blood samples for health monitoring, when previously this was unachievable using standard venous phlebotomy and routine testing processes.