Incorporating Patient Centric Sampling into Multicentre Clinical Trials
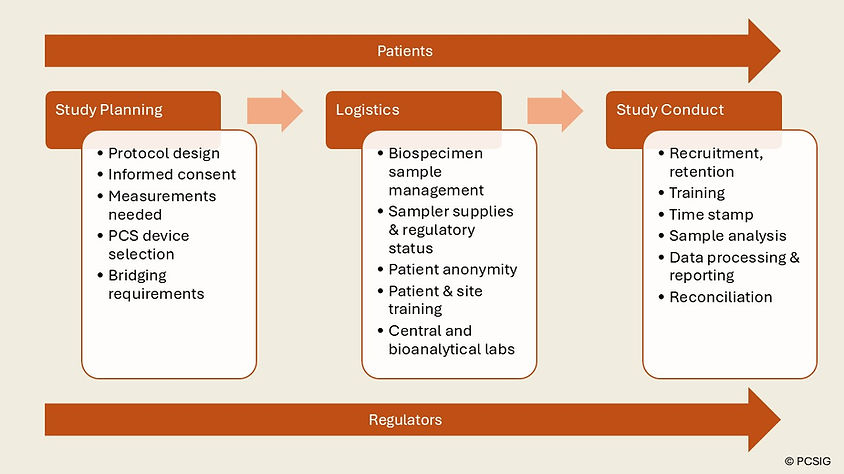
PCSIG convened a two-day workshop at Roche’s facilities in Welwyn Garden City, UK on 11 and 12 June 2024. The event was attended by individuals from organisations representing patient engagement, pharmaceutical and biotech companies, contract research organisations, technology and solutions vendors, publishing and consulting sectors. The purpose of the workshop was to: Outline the key stages associated with the implementation of PCS for multicentre clinical trials; Define the challenges to implementation of PCS for multicentre clinical trials; Identify how we can overcome these challenges.
The output from this event and ongoing activities are available via this link.
