Clinical Trials
This page contains a number of resources for those wishing to use PCS in clinical trials.
The content is intended for guidance only. If you have any recommendations for the content on this page please let us know at contact@pcsig.org.
PCSIG / PBSS Event – Patient Centric Blood Sampling for Facilitating De-Centralized Clinical Trials: Implications for ClinOps, PK / ClinPharm, Bioanalytical and Biomarkers
PBSS and PCSIG ran a joint digital event on 17th October 2024. The event was hosted by Robyn Rourick (Genentech) and Neil Spooner (PCSIG). A range of speakers presented their thoughts on the challenges and successes for implementation of patient centric blood sampling approaches for pharmaceutical clinical trials.
The speakers were Neil Spooner (PCSIG), Mitch Johnson (Veloxity Labs), Katty Wan (Pfizer), Sally Fischer (Genentech), Brian Booth (FDA), Jeff Silverman (Edgewise Therapeutics) and Jeff Plomley (Altasciences).
Follow the link to the recording of the event on the PCSIG YouTube Channel
Report from the PCSIG Patient Advisory Group
PCSIG partnered with MediPaCe to host an on-line workshop with the PCSIG Patient Advisory Group in August 2024, with the aim of improving understanding of the needs & wants of patients experiencing sampling.
People
- 7 patients and caregivers from the UK, Europe & USA, with a range of experience in trials, healthcare systems, health monitoring needs, patient organisations
- Diverse range of conditions included rare, genetic, neurodegenerative, hormonal & inflammatory diseases
Process
Perspectives and insights were gathered on:
- Experiences of providing samples
- Impact of sampling on patients
- Ideas of what’s needed to make sampling easier
- How patients would like to interact with PCSIG in the future
PCSIG Webinar on Demand – Incorporating Patient Centric Sampling into Multicenter Clinical Trials: Summary of a PCSIG Workshop
This webinar presents materials arising from a PCSIG workshop held in June 2024. The presenters discuss the challenges associated with incorporating PCS into multicentre clinical trials, and outline some of the solutions proposed by the workshop attendees, along with resulting actions and outputs.
Follow the link to the recording of the event on the PCSIG YouTube Channel
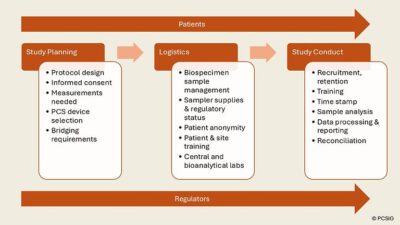
Proceedings from PCSIG Workshop – Incorporating Patient Centric Sampling into Multicentre Clinical Trials
This document describes the output from the workshop held at Roche’s facilities in Welwyn Garden City, UK on 11 and 12 June 2024 attended by 48 individuals representing pharmaceutical and biotech companies, contract research organisations, technology and solutions vendors, academia, publishing and consultants.
The report content includes discussion around a number of challenges encountered when incorporating PCS into clinical trials and suggestions on how to overcome these and potential future activities.
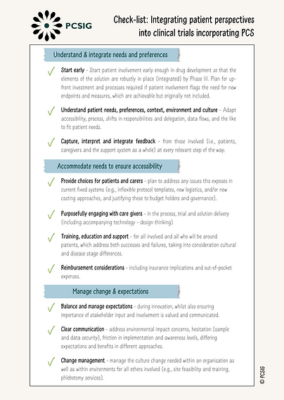
Check-list: Integrating Patient Perspectives into Clinical Trials Incorporating PCS
This document outlines a number of considerations to ensure that the voice of the patient is central when designing and performing clinical trials, particularly when a PCS aspect is included. Use this document as an essential part of designing and running clinical trials and adapting to rising patient needs.
Incorporating Patient Centric Sampling into Multicentre Clinical Trials – Protocol Template Text
This document gives suggested template text for incorporation into study protocol documents when PCS is incorporated into clinical trials.
Incorporating Patient Centric Sampling into Multicentre Clinical Trials – Lab Manual Template Text
This document gives suggested template text for incorporation into study lab manual documents when PCS is incorporated into clinical trials.